Identify Osteoarticular Infection-Causing Bacteria Directly from Clinical Sample!
Mobidiag’s new Prove-it® Bone&Joint assay is CE-IVD marked! The assay provides highly multiplexed and accurate DNA-based identification of directly from a clinical sample – synovial fluid, bone biopsy or tissue sample. All pathogens are covered in a single assay.
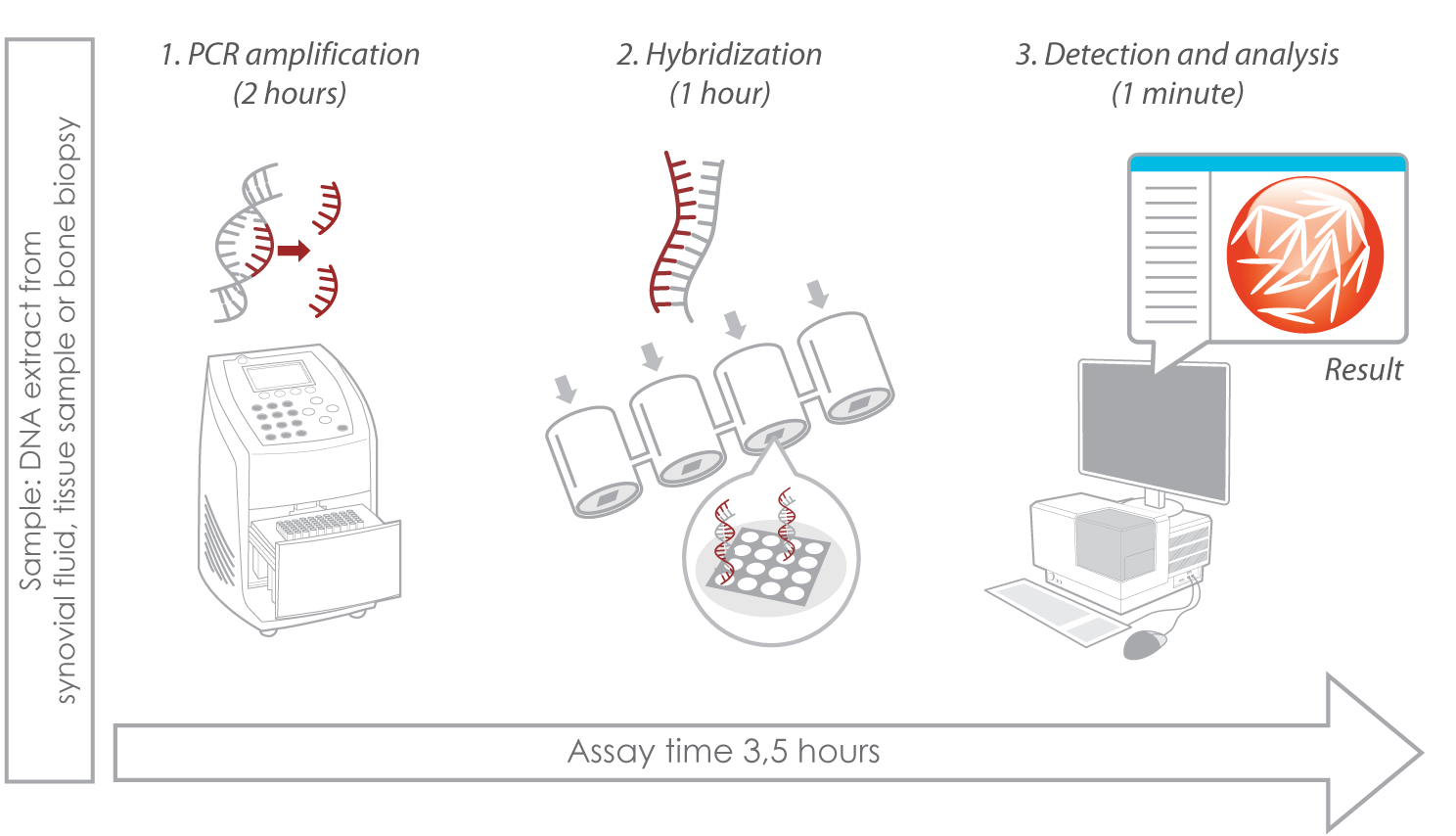
- Assay principle: pathogen DNA amplification in broad-range bacterial PCR followed by identification on a microarray with unique DNA capture probes. Read more about Prove-it technology here.
- Starting material: extracted microbial DNA.
- CE-IVD marked sample types: bone biopsy, tissue or synovial fluid.
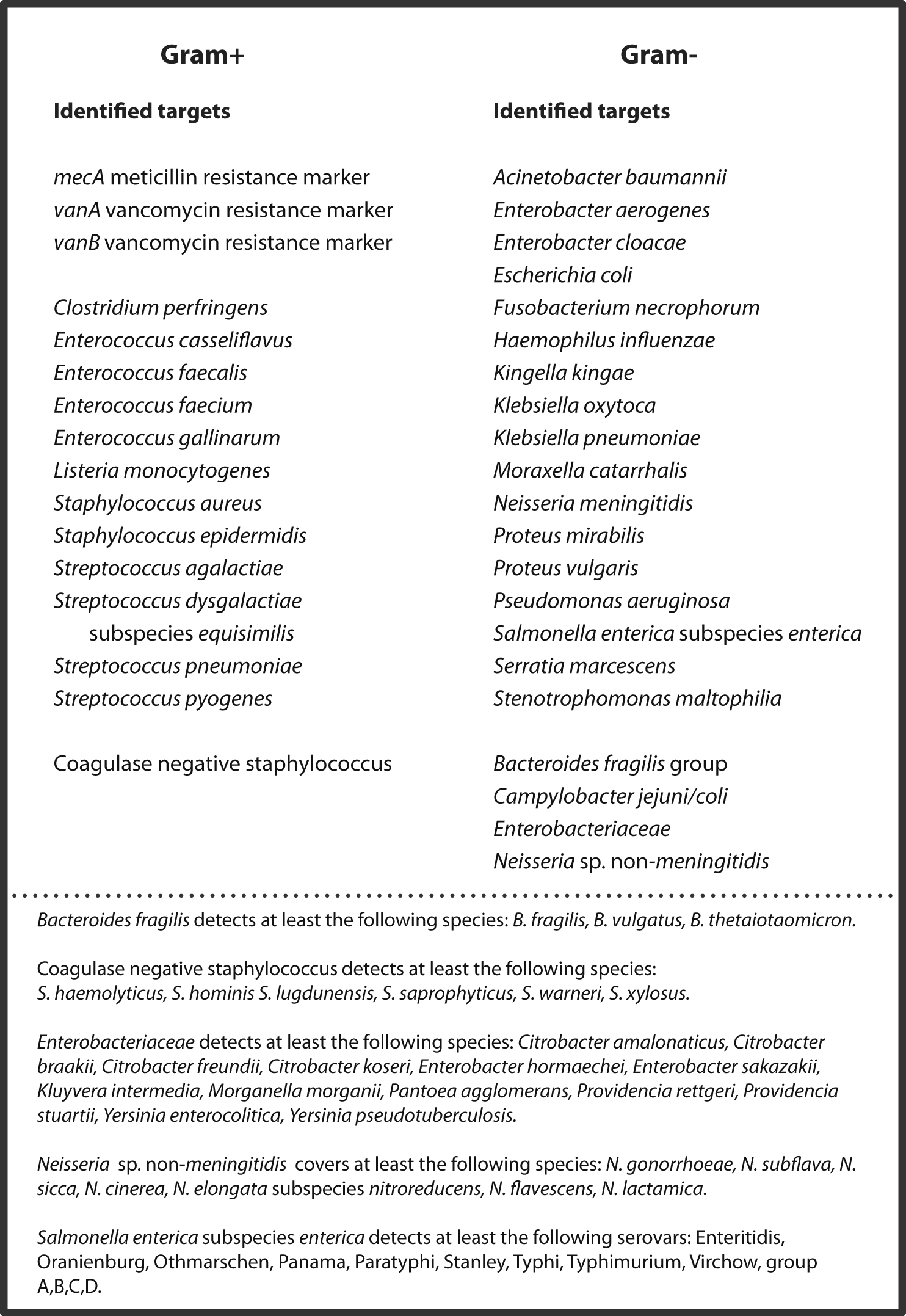
- Assay coverage: 60 bacteria and mecA, vanA and vanB markers for methicillin and vancomycin resistance. View all species included in the pathogen panel here.
- The assay was evaluated in a multicenter clinical trial with over 200 patients, yielding sensitivity and specificity of 91% and 90%, respectively, when compared to routine culturing and 16S rDNA PCR methods.
- Assay platform: Prove-it StripArray.
- Assay time: 3,5 hours from extracted DNA.
- Result interpretation: Automated detection, analysis and result reporting on software guided Prove-it StripArray System. Objective results guaranteed with very sophisticated and easy-to-use Prove-it Advisor software.